clinical trial malaysia
As one of the key players in attaining our national vision the National Pharmaceutical Control. Conducting Clinical Trials in Malaysia.

Hospital Serdang Clinical Research Malaysia
Our specialists are here to help.
. Before you apply to seek a clinical trial license for your products in Malaysia there are some bases you need to cover. 7 A GUIDE TO CONDUCTING CLINICAL TRIALS IN MALAYSIA 7 A GUIDE TO CONDUCTING CLINICAL TRIALS IN MALAYSIA 1 Foreword by Minister of Health Malaysia The total drug discovery and development market size in the top seven Asian countries was estimated at 53 billion in 2011 and is forecast to reach 173 billion by the end of 2018. Photo sharing platform Instagram is starting to trial a tool that relies on artificial intelligence AI to confirm the age of.
In most cases the smaller Asian countries will not require local clinical studies and will accept foreign clinical trial data during the registration process for both medical devices and pharmaceuticals. Malaysian Guideline for Application of Clinical Trial Import Licence and Clinical Trial Exemption Bahagian Regulatori Farmasi Negara NPRA Ministry of Health Malaysia Lot 36 Jln Profesor Diraja Ungku Aziz 46200 Petaling Jaya Selangor Darul Ehsan. This guideline is part of a much bigger initiative of the Phase I Realization Project P1RP that aims to build a complete and comprehensive early phase clinical research ecosystem in the country.
CTIL and Clinical Trial Exemption CTX 5th Edition in 2009 we have witnessed robust growth in clinical research industry with the aim to achieve at least 1000 clinical trials to generate GNI of RM5784 million by the year 2020 in Malaysia. Professional interpretation of these guidelines based on current local existing acts and regulations is required and proper judgment should be exercised in specific situationsclinical trials. ISPE Malaysia Affiliate is delighted to invite you to tune into our upcoming webinar on the topic.
The Requirements Current Trends. Section 26 of the Act empowers the Minister of Health to im pose regulations with respect to drugs including. This study seeks to understand the challenges of managing chronic pain for adults older than 60 years of age who have dementia or memory issues.
The development of Malaysias first Phase I Clinical Trial Guideline marks an important milestone in the history of clinical research in Malaysia. The committee is made up of member representatives and experts from the Ministry of Health various national Universities the Malaysian Pharmaceutical Society the Pharmaceutical. Dr Damenthi Nair.
Challenges of chronic pain management for those with dementia. What is the regulatory authority with oversight for clinical trial in Malaysia. General Clinical Trial.
The Centre for Investigational New Product is the unit in charge. DEFINITION This Guideline adopts the following definitions. Our products are safety and quality assured.
In the present study we aim to evaluate the acceptability and impact of an online program enabling home-based hepatitis C virus HCV self-testing in Malaysia. Since the last publication of Guideline for the application of Clinical Trial Import Licence CTIL and Clinical Trial Exemption CTX 5th Edition in 2009 we have witnessed robust growth in clinical research industry with the aim to achieve at least 1000 clinical trials to generate GNI of RM5784 million by the year 2020 in Malaysia. A properly planned and executed clinical trial is a powerful experimental technique for assessing the effectiveness of an intervention.
Singapore has 43 million people high-quality facilities and highly educated doctors many of whom went to school in the US. Or Europe especially England. PUTRAJAYA 6th April 2020 The Solidarity Trial launched by the World Health Organization WHO will see Malaysias involvement in an international effort to test several drugs in treating COVID-19.
31 Clinical Trial - in which the objective of the trialresearch is of essentially diagnostic or therapeutic value to the patient. The WHO globally coordinated trial is an unprecedented effort to collect reliable data and compare the safety and effectiveness of four treatment protocols. Singapore is a good location for conducting clinical trials because it boasts the second-best healthcare system in Asia after Japan.
For more details please click here. ShaCarri Richardson crashed out of the 100m heats at the US athletics World Championship trials on Thursday as Olympic silver medallist Fred Kerley made a statement in the mens first round. 4 hours agoJune 24 2022 946 AM.
Chronic pain is any pain lasting longer than 3-months such as arthritis pain. The guidelines only give an overview of the conduct of Phase I including FIH trials in Malaysia. On August 2020 the NPRA of Malaysia has updated a document intended to guide the applicant in making Clinical Trial Import Licence CTIL and Clinical Trial Exemption CTX applications to NPRA and reporting to NPRA upon the completion of the clinical trial.
A phase 3 randomized double-blind placebo-controlled clinical trial to study the efficacy and safety of pembrolizumab MK-3475 in Combination With Chemoradiotherapy CRT versus CRT alone in participants with muscle-invasive bladder cancer MIBC. The ministries of health and NPRA have created strict rules to ensure a proper check and balance for allowing clinical trials in Malaysia. You cant just import a product or manufacture one and start its clinical trial.
The current composition of the NCCR was established to ensure that it becomes visionary and pro-active in driving the development of clinical research in the country. Jalan Mewah Utara Pandan Mewah. Hong Kong Indonesia Malaysia the Philippines Singapore Taiwan Thailand and Vietnam.
Gift redemption while stocks last. The primary legislation governing the regulation of clinical trials in Malaysia is the Malaysian Sale of Drugs Act 1952 Act. In Malaysia HIV self-testing has been shown to have moderate to high levels of acceptability depending on the population test used and test delivery framework.
Ad Conduct clinical trials with peace of mind. Trials in Malaysia as their approval is mandatory before a trial can commence. Clinical Research Ward Centre for Clinical Trial CCT Level 7 Hospital Ampang.
603-7883 5400 Second Edition November 1993 Third Edition December 2000. This webinar provides an overview of the Clinical trials requirement and regulations in Malaysia and also the potential of the clinical trial market in the region. Malaysia has a single regulatory authority the National Pharmaceutical Control Bureau NPCB.
Included in the appendices Appendix 4 is a list of. Malaysia Research Clinical Trials RD and Clinical Trials Become a PharmaBoardroom Member for free to access this content Join the 20000 pharmaceutical professionals who already subscribe to PharmaBoardroom.

Home Clinical Update In Covid 19

Institute For Clinical Research Icr Nih My Icr Nih Twitter

Home Clinical Update In Covid 19

Clinical Program Development Pharmalex

Institute For Clinical Research Icr Nih My Icr Nih Twitter
Edc Solutions For Clinical Data Management Openclinica
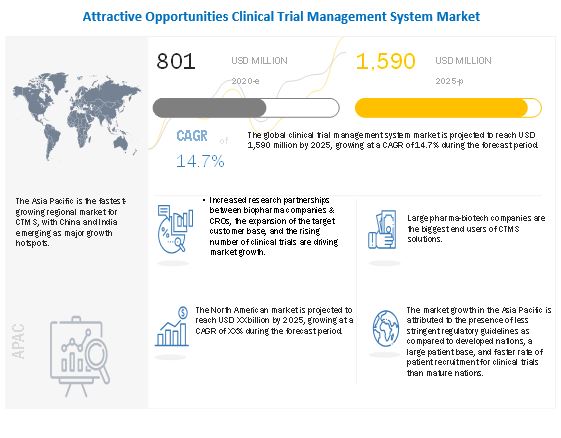
Clinical Trial Management System Market 2022 2025 Size Share And Trends Marketsandmarkets

Breaking Down The Stages Of Clinical Trials For Pharmaceutical Products

Conduct Of Clinical Trials Postmarketing Commitments Clinical Trials Our Science Abbvie

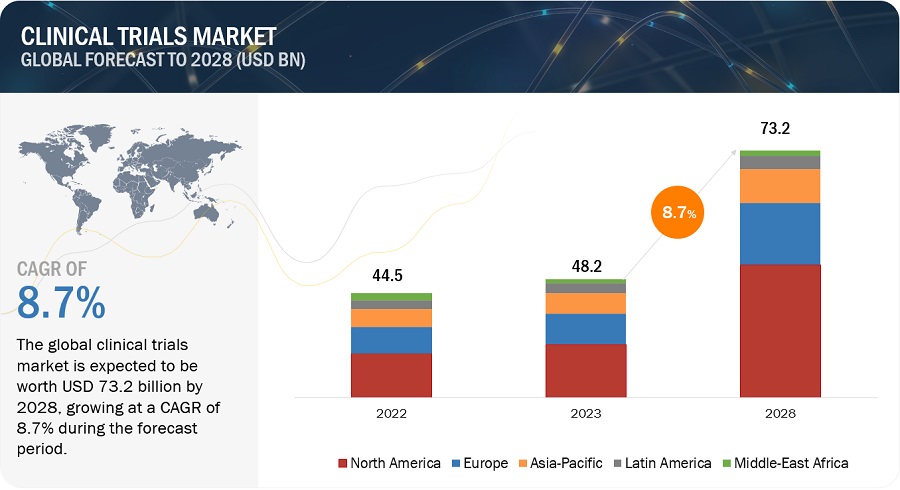
Clinical Trials Market Size Share 2022 2026 Marketsandmarkets

The Progression Of Clinical Trials In Indonesia An Observational Study Of Records From Clinical Trial Registries Databases Sciencedirect
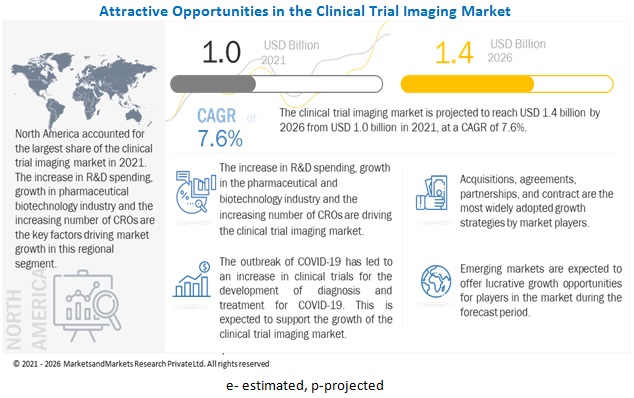
Clinical Trial Imaging Market Global Forecast To 2026 Marketsandmarkets

The Progression Of Clinical Trials In Indonesia An Observational Study Of Records From Clinical Trial Registries Databases Sciencedirect

Clinical Trial Solutions Iqvia

National Medical Research Register

Hospital Ampang Clinical Research Malaysia

The Future Of Clinical Trials In A Post Covid World Pharmaceutical Technology


Comments
Post a Comment